Understanding the Building Blocks of Matter: Ar Protons, Neutrons, Electrons
Have you ever wondered what makes up the atoms that compose everything around us? The answer lies in three fundamental particles: protons, neutrons, and electrons. In this article, we will delve into the properties, roles, and interactions of these particles, providing you with a comprehensive understanding of their significance in the universe.
Protons
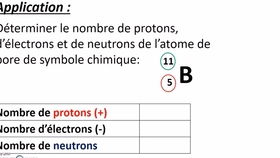
Protons are subatomic particles that carry a positive electric charge. They are located in the nucleus of an atom, which is the central, dense part of the atom. The number of protons in an atom determines its atomic number and, consequently, its chemical identity. For instance, all atoms with one proton are hydrogen atoms, while those with six protons are carbon atoms.
Protons are composed of quarks, specifically two up quarks and one down quark. The up quark has a charge of +2/3, while the down quark has a charge of -1/3. When combined, these quarks result in a net positive charge of +1, which is the charge of a proton. The mass of a proton is approximately 1.6726 x 10^-27 kilograms, making it slightly heavier than an electron.
Neutrons

Neutrons are subatomic particles that have no electric charge, making them neutral. Like protons, they are found in the nucleus of an atom. The number of neutrons in an atom can vary, leading to different isotopes of the same element. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons.
Neutrons are also composed of quarks, specifically one up quark and two down quarks. The up quark has a charge of +2/3, while the down quark has a charge of -1/3. When combined, these quarks result in a net charge of 0, which is the charge of a neutron. The mass of a neutron is approximately 1.6750 x 10^-27 kilograms, slightly heavier than an electron.
Electrons
Electrons are subatomic particles that carry a negative electric charge. Unlike protons and neutrons, electrons are not confined to the nucleus; they orbit around it in regions called electron shells or orbitals. The number of electrons in an atom is equal to the number of protons, ensuring that the atom remains electrically neutral.
Electrons are composed of a single up quark and no down quarks, resulting in a net charge of -1. The mass of an electron is approximately 9.1094 x 10^-31 kilograms, making it much lighter than a proton or neutron. Despite their small mass, electrons play a crucial role in chemical reactions and the formation of chemical bonds.
Table: Properties of Protons, Neutrons, and Electrons
| Particle | Charge | Mass (kg) | Location |
|---|---|---|---|
| Proton | Positive | 1.6726 x 10^-27 | Nucleus |
| Neutron | Neutral | 1.6750 x 10^-27 | Nucleus |
| Electron | Negative | 9.1094 x 10^-31 | Orbiting the nucleus |
Understanding the properties and interactions of protons, neutrons, and electrons is crucial for comprehending the behavior of atoms and molecules. These particles are responsible for the chemical reactions that sustain life, the formation of stars, and the structure of the universe. By unraveling the mysteries of these fundamental particles, scientists continue to expand our knowledge of the cosmos and the intricate fabric of matter.
